Peptides & Peptide Synthesis Products
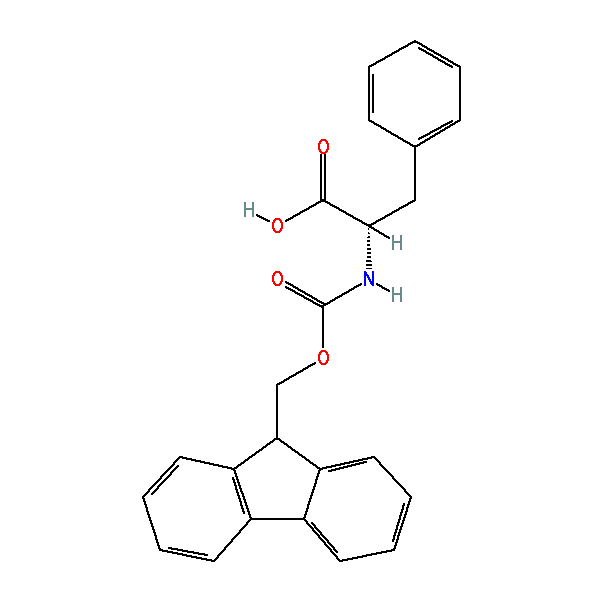
Fmoc-Phe-OH
Catalog No: 41013
Nα-Fmoc-L-phenylalanine, CAS: 35661-40-6, MW: 387.43, Formula: C24H21NO4
Nα-Fmoc-L-phenylalanine, CAS: 35661-40-6, MW: 387.43, Formula: C24H21NO4
$5.00
SKU
41013
Nα-Fmoc-L-phenylalanine
| Catalog Number | 41013 |
|---|---|
| CAS | 35661-40-6 |
| M.W. | 387.43 |
| Formula | C24H21NO4 |
| IUPAC Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-phenylpropanoic acid |
| Synonym | Nα-Fmoc-L-phenylalanine |
| Also Known As |
|
| InChIKey | SJVFAHZPLIXNDH-QFIPXVFZSA-N |
| InChI | InChI=1S/C24H21NO4/c26-23(27)22(14-16-8-2-1-3-9-16)25-24(28)29-15-21-19-12-6-4-10-17(19)18-11-5-7-13-20(18)21/h1-13,21-22H,14-15H2,(H,25,28)(H,26,27)/t22-/m0/s1 |
| SMILES | C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)OCC2C3=CC=CC=C3C4=CC=CC=C24 |
Write Your Own Review