Peptides & Peptide Synthesis Products
Boc-Lys-OH
Catalog No: 48068
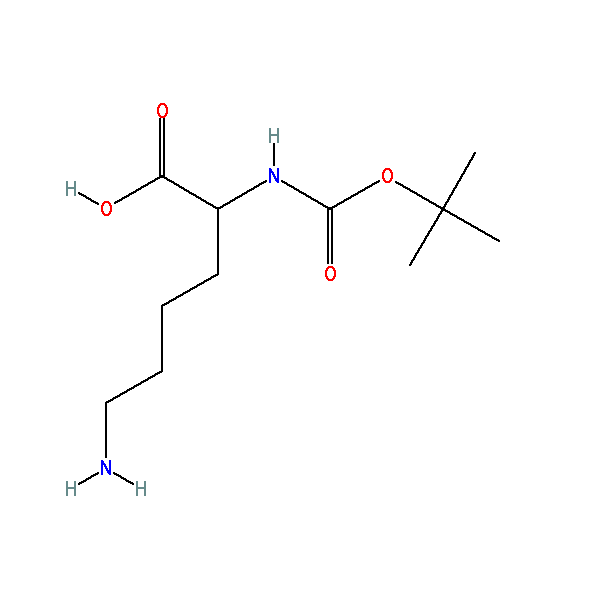
6-amino-2-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid, CAS: 13734-28-6, MW: 246.30, Formula: C11H22N2O4
6-amino-2-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid, CAS: 13734-28-6, MW: 246.30, Formula: C11H22N2O4
$21.00
SKU
48068
6-amino-2-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid
| Catalog Number | 48068 |
|---|---|
| CAS | 13734-28-6 |
| M.W. | 246.30 |
| Formula | C11H22N2O4 |
| IUPAC Name | 6-amino-2-[(2-methylpropan-2-yl)oxycarbonylamino]hexanoic acid |
| Also Known As |
|
| InChIKey | DQUHYEDEGRNAFO-UHFFFAOYSA-N |
| InChI | InChI=1S/C11H22N2O4/c1-11(2,3)17-10(16)13-8(9(14)15)6-4-5-7-12/h8H,4-7,12H2,1-3H3,(H,13,16)(H,14,15) |
| SMILES | CC(C)(C)OC(=O)NC(CCCCN)C(=O)O |
Write Your Own Review