Peptides & Peptide Synthesis Products
Boc-Met-OH
Catalog No: 48084
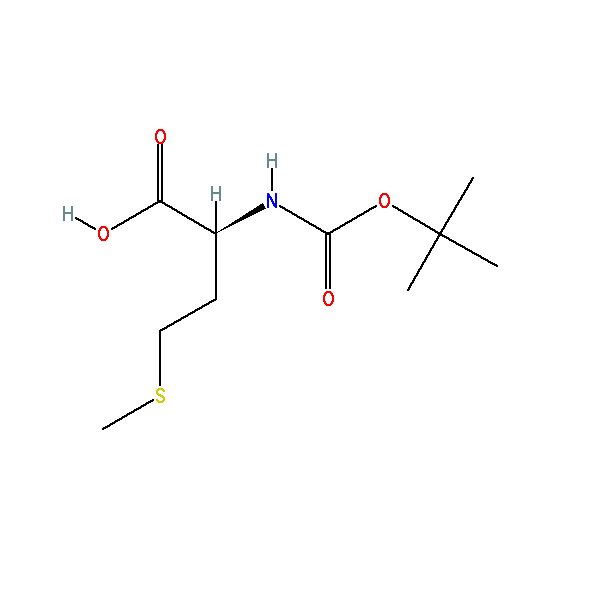
(2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-methylsulfanylbutanoic acid, CAS: 2488-15-5, MW: 249.33, Formula: C10H19NO4S
(2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-methylsulfanylbutanoic acid, CAS: 2488-15-5, MW: 249.33, Formula: C10H19NO4S
$21.00
SKU
48084
(2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-methylsulfanylbutanoic acid
| Catalog Number | 48084 |
|---|---|
| CAS | 2488-15-5 |
| M.W. | 249.33 |
| Formula | C10H19NO4S |
| IUPAC Name | (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-4-methylsulfanylbutanoic acid |
| Also Known As |
|
| InChIKey | IMUSLIHRIYOHEV-ZETCQYMHSA-N |
| InChI | InChI=1S/C10H19NO4S/c1-10(2,3)15-9(14)11-7(8(12)13)5-6-16-4/h7H,5-6H2,1-4H3,(H,11,14)(H,12,13)/t7-/m0/s1 |
| SMILES | CC(C)(C)OC(=O)N[C@@H](CCSC)C(=O)O |
Write Your Own Review